Developmental Genetics
Yoshiki Hayashi(Assis. Prof.)Sato Masanao (Assis. Prof.)
RESEARCH THEMES
Germ cells are the specialized cells that can transmit the genetic materials from one generation to the next in sexual reproduction. All of the other cells of the body are somatic cells. This separation of germ and somatic cells is one of the oldest problems in developmental biology. In many animal groups, a specialized portion of egg cytoplasm, or germ plasm, is inherited by the cell lineage which gives rise to germ cells. This cell lineage is called germline. The germline progenitors eventually migrate into the gonads, where they differentiate as germline stem cells to form eggs and sperm when the organisms are physically matured. We have been focusing on the mechanisms regulating "germness", "sex differences" and "stemness" of the germline.
In Drosophila, germ plasm is localized in the posterior pole region of eggs, and partitioned into the germline progenitors, or pole cells. Several components of germ plasm have been identified in this animal. One of these components is maternal Nanos (nos) protein. Nos is inherited by pole cells at the blastoderm stage and is detectable in these cells throughout embryogenesis. In the absence of maternal Nos, pole cells undergo somatic differentiation, showing that Nos is essential for establishing germ/soma dichotomy. Furthermore, we also attempted to identify genes expressed in pole cells and/or in somatic cells within the gonad by a comprehensive approach. Based on these data, we are now examining the mechanism regulating sex differences of pole cells and germline stem cell formation.
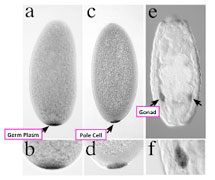
Figure
Germline development in Drosophila embryos.
Germ plasm (a and b; cleavage embryo) is partitioned into pole cells (c and d; blastodermal embryo). Pole cells migrate into the gonads to differentiate in germ cells (e and f; late embryo).
SELECTED PUBLICATIONS
- Y. Kitadate, S. Shigenobu, K. Arita and S. Kobayashi (2007) Boss/Sev signaling from germline to soma restricts germline-stem-cell-niche formation in the anterior region of Drosophila male gonad. Dev. Cell, 13, 151-159.
- K. Sato, Y. Hayashi, Y. Ninomiya, S. Shigenobu, K. Arita, M. Mukai and S. Kobayashi (2007) Maternal Nanos represses hid/skl-dependent apoptosis to maintain the germ line in Drosophila embryos. Proc. Natl. Acad. Sci., USA., 104, 7455-7460.
- S. Shigenobu, Y. Kitadate, C. Noda and S. Kobayashi (2006) Molecular characterization of embryonic gonads by gene expression profiling in Drosophila melanogaster. Proc. Natl. Acad. Sci., USA., 103, 13728-13733.
- T. Sengoku, O. Nureki, A. Nakamura, S. Kobayashi and S. Yokoyama (2006) Structural Basis for RNA Unwinding by the DEAD-Box Protein Drosophila Vasa. Cell 125, 287-300.
- Y. Hayashi, M. Hayashi and S. Kobayashi (2004) Nanos suppresses somatic cell fate in Drosophila germline. Proc. Natl. Acad. Sci. USA. 101, 10338-10342.
- M. Tsuda, Y. Sasaoka, M. Kiso, K. Abe, S. Haraguchi, S. Kobayashi and Y. Saga: Conserved role of nanos proteins in germ cell development. Science 301, 1239-1241 (2003)
- S. Kobayashi, M. Yamada, M. Asaoka and T. Kitamura: Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature 380, 708-711 (1996)


